Renal Biomarkers Market Size Projected to Reach USD 2.83 Billion by 2032
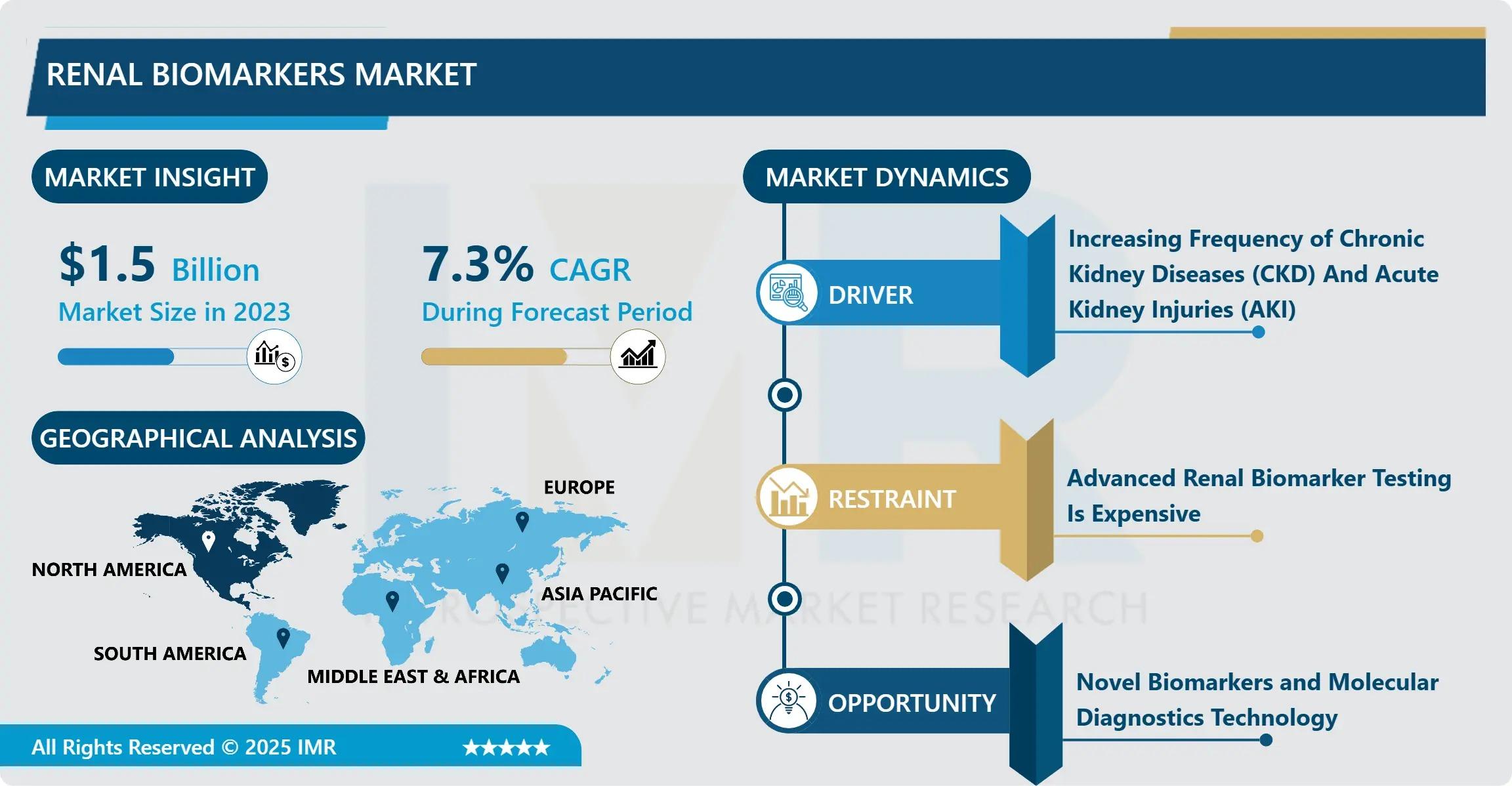
According to a new report published by Introspective Market Research, Renal Biomarkers Market by Biomarker Type (Functional Biomarkers, Up-regulated Proteins, Others), Diagnostic Technique (ELISA, PETIA, Colorimetric Assay, CLIA, Others), and End User (Hospitals, Diagnostic Laboratories, Academic & Research Institutes), The Global Renal Biomarkers Market Size Was Valued at USD 1.5 Billion in 2023 and is Projected to Reach USD 2.83 Billion by 2032, Growing at a CAGR of 7.3%.
Introduction / Market Overview:
The global renal biomarkers market is a cornerstone of modern nephrology, providing essential tools for the early detection, diagnosis, and monitoring of kidney-related diseases. Renal biomarkers are biological molecules—typically proteins or enzymes—found in blood or urine that indicate the functional health of the kidneys. Traditional markers like serum creatinine have long been the standard; however, the market is rapidly shifting toward more sensitive, "up-regulated" proteins such as NGAL and KIM-1. These advanced biomarkers offer the distinct advantage of detecting Acute Kidney Injury (AKI) and Chronic Kidney Disease (CKD) hours or even days earlier than traditional methods, allowing for life-saving clinical interventions.
In an era of rising chronic conditions, renal biomarkers serve as critical indicators for major industries, including pharmaceuticals and clinical diagnostics. They are extensively used in drug development to monitor for drug-induced kidney injury and in hospitals to manage critically ill patients at risk of organ failure. By providing a non-invasive and highly accurate window into renal health, these biomarkers facilitate a move away from "wait-and-see" approaches toward proactive, personalized patient management. As healthcare systems globally grapple with an aging population and high rates of diabetes and hypertension, the integration of these sophisticated diagnostic tools is becoming a clinical necessity.
Market Segmentation:
The Renal Biomarkers Market is segmented into Biomarker Type, Diagnostic Technique, and End User. By Biomarker Type, the market is categorized into (Functional Biomarkers, Up-regulated Proteins, and Others). By Diagnostic Technique, the market is categorized into (ELISA, Particle-Enhanced Turbidimetric Immunoassay (PETIA), Colorimetric Assay, Chemiluminescent Enzyme Immunoassay (CLIA), and Others). By End User, the market is categorized into (Hospitals, Diagnostic Laboratories, and Academic & Research Institutes).
Growth Driver:
The primary driver for the Renal Biomarkers Market is the soaring global prevalence of chronic kidney disease (CKD) fueled by rising rates of diabetes and hypertension. As the leading causes of renal failure, these metabolic conditions require continuous monitoring to prevent end-stage renal disease (ESRD). Healthcare providers are increasingly adopting biomarker-based screening to stratify patient risk and slow disease progression. Furthermore, the growing geriatric population—who are biologically more prone to renal dysfunction—is creating a consistent and expanding patient base, compelling healthcare systems to invest in high-sensitivity diagnostic assays that can detect sub-clinical kidney damage early.
Market Opportunity:
A significant market opportunity lies in the integration of AI-driven diagnostics and point-of-care (POC) testing platforms. The development of handheld or bedside devices that can provide real-time renal biomarker results would revolutionize critical care and rural healthcare settings. Companies that can combine multiplex biomarker panels with artificial intelligence algorithms to predict "risk scores" for kidney failure have a unique opportunity to lead the market. By transforming complex biomarker data into actionable clinical insights at the bedside, manufacturers can meet the urgent demand for faster, decentralized testing that reduces hospital stay durations and improves overall patient outcomes.
Detailed Segmentation:
Title: Renal Biomarkers Market, Segmentation The Renal Biomarkers Market is segmented on the basis of Biomarker Type, Diagnostic Technique, and End User.
Biomarker Type The Biomarker Type segment is further classified into Functional Biomarkers and Up-regulated Proteins. Among these, the Functional Biomarkers sub-segment accounted for the highest market share in 2023. This dominance is due to the deep-rooted clinical reliance on established markers like serum creatinine, blood urea nitrogen (BUN), and urine albumin. These tests are highly cost-effective, universally standardized, and integrated into almost all routine chemistry panels globally. Their role as the first line of defense in kidney function assessment ensures a high-volume, recurring demand across all levels of the healthcare system.
Diagnostic Technique The Diagnostic Technique segment is further classified into ELISA, CLIA, and Colorimetric Assays. Among these, the Enzyme-Linked Immunosorbent Assay (ELISA) sub-segment accounted for the highest market share in 2023. ELISA remains the gold standard for biomarker quantification due to its exceptional sensitivity and specificity, particularly for detecting low-concentration proteins like NGAL. Its widespread availability in both commercial diagnostic kits and academic research settings, combined with a robust existing infrastructure of ELISA plate readers in laboratories worldwide, makes it the primary technique for validating and routine-testing renal biomarkers.
Some of The Leading/Active Market Players Are-
- Thermo Fisher Scientific Inc. (USA)
- Abbott Laboratories (USA)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Siemens Healthineers AG (Germany)
- Danaher Corporation (Beckman Coulter) (USA)
- Bio-Rad Laboratories, Inc. (USA)
- bioMérieux SA (France)
- Randox Laboratories Ltd. (UK)
- BioPorto A/S (Denmark)
- SphingoTec GmbH (Germany)
- Sysmex Corporation (Japan)
- QIAGEN N.V. (Netherlands)
- DiaSorin S.p.A. (Italy)
- Ortho Clinical Diagnostics (QuidelOrtho) (USA)
- Boditech Med Inc. (South Korea)
and other active players.
Key Industry Developments
News 1: In May 2025, Boditech Med Inc. and SphingoTec GmbH announced the commercial launch of the AFIAS penKid assay, a novel diagnostic tool designed for real-time kidney function assessment in acute care settings. The penKid biomarker measures proenkephalin, a marker that is not affected by inflammation, providing a more accurate reflection of the glomerular filtration rate (GFR). This launch represents a major step toward providing clinicians with reliable, bedside tools to detect acute kidney injury in septic and critically ill patients, potentially reducing mortality rates in the ICU.
News 2: In September 2025, Baxter International Inc. entered into a significant distribution agreement with bioMérieux to market the NEPHROCLEAR™ CCL14 diagnostic test in the United States and other global markets. The CCL14 biomarker is specifically used to predict the persistence of severe acute kidney injury, helping physicians identify which patients are likely to require renal replacement therapy. This partnership leverages Baxter’s extensive footprint in renal care and bioMérieux’s expertise in immunoassay technology to improve the management of severe AKI cases globally.
Key Findings of the Study
- Dominant Segments: Functional Biomarkers and ELISA techniques lead the market due to cost-efficiency and established clinical trust.
- Leading Regions: North America holds the largest share (~47%), while Asia-Pacific is projected to be the fastest-growing region.
- Key Growth Drivers: Rising incidence of diabetic nephropathy and increasing geriatric populations globally.
- Market Trends: Shift toward "up-regulated" protein panels and the adoption of AI-enabled predictive analytics in nephrology.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
